Activated Carbon and Ozone to Reduce Simazine in Water
Abstract
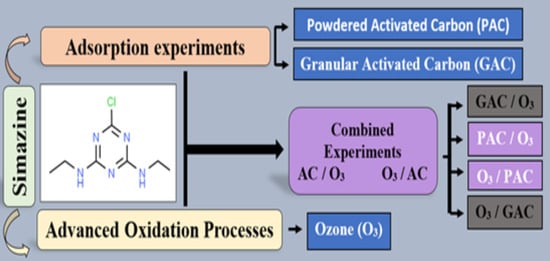
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Activated Carbon
2.3. Characterisation of Activated Carbons
2.4. Analytical Method
2.5. Adsorption Experiments
2.6. Oxidation Experiments
2.7. Combined Experiments
3. Results and Discussion
3.1. Characterisation of the Pulsorb PWX HA and Calgon Filtrasorb 400
3.2. Simazine Adsorption with Powdered Activated Carbon
3.3. Simazine Adsorption with Granular Activated Carbon
3.4. Comparison of PAC versus GAC
3.5. Simazine Oxidation with Ozone
3.6. Combined PAC/O3 and O3/PAC Experiments
3.7. Combined GAC/O3 and O3/GAC Experiments
3.8. Comparison AC/O3 versus O3/AC
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- European Commission. Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 Amending Directives 2000/60/EC and 2008/105/EC as Regards Priority Substances in the Field of Water Policy. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32013L0039&from=EN (accessed on 25 June 2020).
- García, J.; García-Galán, M.J.; Day, J.W.; Boopathy, R.; White, J.R.; Wallace, S.; Hunter, R.G. A review of emerging organic contaminants (EOCs), antibiotic resistant bacteria (ARB), and antibiotic resistance genes (ARGs) in the environment: Increasing removal with wetlands and reducing environmental impacts. Bioresour. Technol. 2020, 307, 123228. [Google Scholar] [CrossRef] [PubMed]
- Kibuye, F.A.; Gall, H.E.; Veith, T.L.; Elkin, K.R.; Elliott, H.A.; Harper, J.P.; Watson, J.E. Influence of hydrologic and anthropogenic drivers on emerging organic contaminants in drinking water sources in the Susquehanna River Basin. Chemosphere 2020, 245, 125583. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, W.L.; He, H.; Yang, C.; Yu, J.; Wu, X.; Zeng, G.; Tarre, S.; Green, M. Preparation, performances and mechanisms of magnetic Saccharomyces cerevisiae bionanocomposites for atrazine removal. Chemosphere 2018, 200, 380–387. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Wu, B.; Yang, C. Effects of fulvic acids and electrolytes on colloidal stability and photocatalysis of nano-TiO2 for atrazine removal. Int. J. Environ. Sci. Technol. 2018, 16, 7275–7284. [Google Scholar] [CrossRef]
- Koblížek, M.; Malý, J.; Masojídek, J.; Komenda, J.; Kucera, T.; Giardi, M.T.; Mattoo, A.K.; Pilloton, R. A biosensor for the detection of triazine and phenylurea herbicides designed using Photosystem II coupled to a screen-printed electrode. Biotechnol. Bioeng. 2002, 78, 110–116. [Google Scholar] [CrossRef]
- Lipsey, R.L.; Ware, G. The Pesticide Book. Fla. Èntomol. 1979, 62, 284. [Google Scholar] [CrossRef]
- Hernã¡ndez, M.; Villalobos, P.; Morgante, V.; Gonzã¡lez, M.; Reiff, C.; Moore, E.; Seeger, M.; Meharg, C.; Morgante, V. Isolation and characterization of a novel simazine-degrading bacterium from agricultural soil of central Chile, Pseudomonassp. MHP41. FEMS Microbiol. Lett. 2008, 286, 184–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanderson, J.T.; Letcher, R.J.; Heneweer, M.; Giesy, J.P.; Berg, M.V.D. Effects of chloro-s-triazine herbicides and metabolites on aromatase activity in various human cell lines and on vitellogenin production in male carp hepatocytes. Environ. Heal. Perspect. 2001, 109, 1027–1031. [Google Scholar] [CrossRef]
- Zorrilla, L.M.; Gibson, E.K.; Stoker, T.E. The effects of simazine, a chlorotriazine herbicide, on pubertal development in the female Wistar rat. Reprod. Toxicol. 2010, 29, 393–400. [Google Scholar] [CrossRef]
- Gammon, D.W.; Aldous, C.N.; Carr, W.C.; Sanborn, J.R.; Pfeifer, K.F. A risk assessment of atrazine use in California: Human health and ecological aspects. Pest. Manag. Sci. 2005, 61, 331–355. [Google Scholar] [CrossRef]
- Tappe, W.; Groeneweg, J.; Jantsch, B. Diffuse atrazine pollution in German aquifers. Biogeochemistry 2002, 13, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Strandberg, M.; Scott-Fordsmand, J.J. Field effects of simazine at lower trophic levels—A review. Sci. Total. Environ. 2002, 296, 117–137. [Google Scholar] [CrossRef]
- Koutnik, D.; Stara, A.; Velisek, J. The effect of selected triazines on fish: A review. Slov. Vet. Res. 2015, 52, 107–131. [Google Scholar]
- Kim, H.; Homan, M. Evaluation of pharmaceuticals and personal care products (PPCPs) in drinking water originating from Lake Erie. J. Great Lakes Res. 2020. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Zheng, L.; Lu, S.; Yan, Z.; Ling, J. Occurrence, distribution and ecological risk assessment of the herbicide simazine: A case study. Chemosphere 2018, 204, 442–449. [Google Scholar] [CrossRef]
- USEPA EPA-HQ-OPP-2013-0251. Available online: https://www.epa.gov/sites/production/files/2019-12/documents/simazine_pid_signed.pdf. (accessed on 25 June 2020).
- European Commission Health a Consumer Protection Directorate-General Simazine. SANCO/10495/2003-rev. Final. Review Report for the Active Substance Simazine. Available online: http://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/public/?event=activesubstance.ViewReview&id=237 (accessed on 25 June 2020).
- Guan, S.H.; Huang, M.W.; Li, X.; Cai, Q. Determination of atrazine, simazine, alachlor, and metolachlor in surface water using dispersive pipette extraction and gas chromatography-Mass spectrometry. Anal. Lett. 2018, 51, 613–625. [Google Scholar] [CrossRef]
- Metcalf, W.; Eddy, C. Metcalf and Eddy Wastewater engineering: Treatment and reuse. Wastewater Eng. Treat. Reuse 2003, 384. [Google Scholar]
- Novotny, V. Water Quality: Diffuse Pollution and Watershed Management; John Wiley & Sons: Hoboken, NJ, USA, 2003; ISBN 0471396338. [Google Scholar]
- Shanmuganathan, S.; Loganathan, P.; Kazner, C.; Johir, M.; Vigneswaran, S. Submerged membrane filtration adsorption hybrid system for the removal of organic micropollutants from a water reclamation plant reverse osmosis concentrate. Desalination 2017, 401, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Bueno, M.M.; Gomez, M.; Herrera, S.; Hernando, M.; Agüera, A.; Fernández-Alba, A. Occurrence and persistence of organic emerging contaminants and priority pollutants in five sewage treatment plants of Spain: Two years pilot survey monitoring. Environ. Pollut. 2012, 164, 267–273. [Google Scholar] [CrossRef]
- Bataller, M.O.; Fernández, L.A.; Véliz, E. Eficiencia y sostenibilidad del empleo del ozono en la gestión de los recursos hidrícos. Rev. Int. Contam. Ambient. 2010, 26, 85–95. [Google Scholar]
- USEPA Basic Information about Atrazine in Drinking Water. Available online: https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations (accessed on 25 June 2020).
- Lladó, J.; Lao-Luque, C.; Ruiz, B.; Fuente, E.; Solé-Sardans, M.; Dorado, A.D. Role of activated carbon properties in atrazine and paracetamol adsorption equilibrium and kinetics. Process. Saf. Environ. Prot. 2015, 95, 51–59. [Google Scholar] [CrossRef]
- Gardi, I.; Nir, S.; Mishael, Y.G. Filtration of triazine herbicides by polymer-clay sorbents: Coupling an experimental mechanistic approach with empirical modeling. Water Res. 2015, 70, 64–73. [Google Scholar] [CrossRef]
- Zhang, W.; Ruan, G.; Li, X.; Jiang, X.; Huang, Y.; Du, F.; Li, J. Novel porous carbon composites derived from a graphene-modified high-internal-Phase emulsion for highly efficient separation and enrichment of triazine herbicides. Anal. Chim. Acta 2019, 1071, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, E.F.; Andriantsiferana, C.; Wilhelm, A.-M.; Delmas, H. Competitive adsorption of phenolic compounds from aqueous solution using sludge-based activated carbon. Environ. Technol. 2011, 32, 1325–1336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bueno, M.B.-R.D.H.; Boluda-Botella, N.; Rico, D.P. Removal of emerging pollutants in water treatment plants: Adsorption of methyl and propylparaben onto powdered activated carbon. Adsorpion 2019, 25, 983–999. [Google Scholar] [CrossRef]
- Di Bernardo, L.; Dantas, Ângela, D.B. Métodos e técnicas de tratamento de água. Eng. Sanit. e Ambient. 2006, 11, 107. [Google Scholar] [CrossRef] [Green Version]
- Cañizares, P.; Lobato, J.; Paz, R.; Rodrigo, M.A.; Sáez, C. Advanced oxidation processes for the treatment of olive-oil mills wastewater. Chemosphere 2007, 67, 832–838. [Google Scholar] [CrossRef]
- Lucas, M.S.; Peres, J.A.; Puma, G.L. Treatment of winery wastewater by ozone-based advanced oxidation processes (O3, O3/UV and O3/UV/H2O2) in a pilot-scale bubble column reactor and process economics. Sep. Purif. Technol. 2010, 72, 235–241. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, Z.; Zhang, R.-H.; Zheng, C.; Zhang, H.; Qiu, Y.; Zhao, J. Extraction of copper from sewage sludge using biodegradable chelant EDDS. J. Environ. Sci. 2008, 20, 970–974. [Google Scholar] [CrossRef]
- Hollender, J.; Zimmermann, S.G.; Koepke, S.; Krauss, M.; McArdell, C.S.; Ort, C.; Singer, H.; Von Gunten, U.; Siegrist, H. Elimination of organic micropollutants in a municipal wastewater treatment plant upgraded with a full-scale post-ozonation followed by sand filtration. Environ. Sci. Technol. 2009, 43, 7862–7869. [Google Scholar] [CrossRef]
- Rizzo, L.; Malato, S.; Antakyali, D.; Beretsou, V.G.; Đolić, M.B.; Gernjak, W.; Heath, E.; Ivancev-Tumbas, I.; Karaolia, P.; Ribeiro, A.R.L.; et al. Consolidated vs new advanced treatment methods for the removal of contaminants of emerging concern from urban wastewater. Sci. Total. Environ. 2019, 655, 986–1008. [Google Scholar] [CrossRef] [PubMed]
- Mathon, B.; Coquery, M.; Liu, Z.; Penru, Y.; Guillon, A.; Esperanza, M.; Miège, C.; Choubert, J.-M. Ozonation of 47 organic micropollutants in secondary treated municipal effluents: Direct and indirect kinetic reaction rates and modelling. Chemosphere 2020, 127969. [Google Scholar] [CrossRef]
- Mathon, B.; Coquery, M.; Miege, C.; Penru, Y.; Choubert, J.-M. Removal efficiencies and kinetic rate constants of xenobiotics by ozonation in tertiary treatment. Water Sci. Technol. 2017, 75, 2737–2746. [Google Scholar] [CrossRef]
- Benner, J.; Salhi, E.; Ternes, T.; Von Gunten, U. Ozonation of reverse osmosis concentrate: Kinetics and efficiency of beta blocker oxidation. Water Res. 2008, 42, 3003–3012. [Google Scholar] [CrossRef]
- Gerrity, D.; Gamage, S.; Jones, D.; Korshin, G.V.; Lee, Y.; Pisarenko, A.N.; Trenholm, R.A.; Von Gunten, U.; Wert, E.C.; Snyder, S.A. Development of surrogate correlation models to predict trace organic contaminant oxidation and microbial inactivation during ozonation. Water Res. 2012, 46, 6257–6272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beltrán, F.; García-Araya, J.F.; Navarrete, V.; Rivas, F.J. An attempt to model the kinetics of the ozonation of simazine in water. Ind. Eng. Chem. Res. 2002, 41, 1723–1732. [Google Scholar] [CrossRef]
- He, H.; Liu, Y.; You, S.; Liu, J.; Xiao, H.; Tu, Z. A review on recent treatment technology for herbicide atrazine in contaminated environment. Int. J. Environ. Res. Public Health 2019, 16, 5129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De los Bernal Romero del Hombre Bueno, M.Á. Reducción De Microcontaminantes Mediante Procesos Biológicos. Ph.D. Thesis, University of Alicante, Alicante, Spain, 2020. [Google Scholar]
- Flores, K.; Valdes, C.; Ramirez, D.; Eubanks, T.; Lopez, J.; Hernandez, C.; Alcoutlabi, M.; Parsons, J. The effect of hybrid zinc oxide/graphene oxide (ZnO/GO) nano-catalysts on the photocatalytic degradation of simazine. Chemosphere 2020, 259, 127414. [Google Scholar] [CrossRef]
- Catalkaya, E.C.; Kargi, F. Advanced oxidation and mineralization of simazine using Fenton’s reagent. J. Hazard. Mater. 2009, 168, 688–694. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/ (accessed on 25 June 2020).
- Wilson, P.C.; Wilson, S.B. Toxicity of the herbicides bromacil and simazine to the aquatic macrophyte, Vallisneria americana Michx. Environ. Toxicol. Chem. 2010, 29, 201–211. [Google Scholar] [CrossRef]
- Van Der Bruggen, B.; Schaep, J.; Maes, W.; Wilms, D.; Vandecasteele, C. Nanofiltration as a treatment method for the removal of pesticides from ground waters. Desalination 1998, 117, 139–147. [Google Scholar] [CrossRef]
- Chen, S.-S.; Taylor, J.S.; Mulford, L.A.; Norris, C.D. Influences of molecular weight, molecular size, flux, and recovery for aromatic pesticide removal by nanofiltration membranes. Desalination 2004, 160, 103–111. [Google Scholar] [CrossRef]
- D28 Committee test method for determination of particle size of powdered activated carbon by air jet sieving. ASTM Int 2015, 15, 3. [CrossRef]
- American water works association granular activated carbon. Am. Soc. Test. Mater. 2012, 56. [CrossRef]
- ASTM, D.-06 ASTM D1193-06 2018. Standard Specification for Reagent Water. ASTM Int. 2018, 6. [Google Scholar] [CrossRef]
- ASTM, D.-98 ASTM D3860-98 2014. Standard practice for determination of adsorptive capacity of activated carbon by a micro-isotherm technique for adsorbates at ppb concentrations. ASTM Int. 2014, 15, 4. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Flores Baquero, O. Design of an Activated Carbon Columns Plant. for Studying Micro-Pollutants Removal. Available online: http://bibing.us.es/proyectos/abreproy/20087/fichero (accessed on 25 June 2020).
- Li, Y.; Du, Q.; Liu, T.; Sun, J.; Jiao, Y.; Xia, Y.; Xia, L.; Wang, Z.; Zhang, W.; Wang, K.; et al. Equilibrium, kinetic and thermodynamic studies on the adsorption of phenol onto graphene. Mater. Res. Bull. 2012, 47, 1898–1904. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, X.; Wu, Y.; Huang, H.; Zeng, G.; Liu, Y.; Wang, X.; Lin, N.; Qi, Y. Adsorption characteristics and behaviors of graphene oxide for Zn (II) removal from aqueous solution. Appl. Surf. Sci. 2013, 279, 432–440. [Google Scholar] [CrossRef]
- Matsui, Y.; Fukuda, Y.; Inoue, T.; Matsushita, T. Effect of natural organic matter on powdered activated carbon adsorption of trace contaminants: Characteristics and mechanism of competitive adsorption. Water Res. 2003, 37, 4413–4424. [Google Scholar] [CrossRef]
- Jiang, H.; Adams, C. Treatability of chloro-s-triazines by conventional drinking water treatment technologies. Water Res. 2006, 40, 1657–1667. [Google Scholar] [CrossRef] [PubMed]
- Moussavi, G.; Khosravi, R. The removal of cationic dyes from aqueous solutions by adsorption onto pistachio hull waste. Chem. Eng. Res. Des. 2011, 89, 2182–2189. [Google Scholar] [CrossRef]
- Nam, S.-W.; Choi, D.-J.; Kim, S.-K.; Her, N.; Zoh, K.-D. Adsorption characteristics of selected hydrophilic and hydrophobic micropollutants in water using activated carbon. J. Hazard. Mater. 2014, 270, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Moussavi, G.; Hossaini, Z.; Pourakbar, M. High-rate adsorption of acetaminophen from the contaminated water onto double-oxidized graphene oxide. Chem. Eng. J. 2016, 287, 665–673. [Google Scholar] [CrossRef]
- Li, C.; Wang, L.; Shen, Y. The removal of atrazine, simazine, and prometryn by granular activated carbon in aqueous solution. Desalin. Water Treat. 2013, 52, 3510–3516. [Google Scholar] [CrossRef]
- Randtke, S.J.; Snoeyink, V.L. Evaluating GAC adsorptive capacity. J. -Am. Water Work. Assoc. 1983, 75, 406–413. [Google Scholar] [CrossRef]
- Choi, K.J.; Kim, S.G.; Kim, C.W.; Kim, S.H. Effects of activated carbon types and service life on removal of endocrine disrupting chemicals: Amitrol, nonylphenol, and bisphenol-A. Chemosphere 2005, 58, 1535–1545. [Google Scholar] [CrossRef]
- Barton, S.; Evans, M.; Halliop, E.; Macdonald, J. Acidic and basic sites on the surface of porous carbon. Carbon 1997, 35, 1361–1366. [Google Scholar] [CrossRef]
- Faria, P.; Órfão, J.; Pereira, M. Activated carbon catalytic ozonation of oxamic and oxalic acids. Appl. Catal. B Environ. 2008, 79, 237–243. [Google Scholar] [CrossRef]
- Faria, P.; Órfão, J.; Pereira, M.F.R. A novel ceria–activated carbon composite for the catalytic ozonation of carboxylic acids. Catal. Commun. 2008, 9, 2121–2126. [Google Scholar] [CrossRef]
- Nahim-Granados, S.; Rivas-Ibáñez, G.; Pérez, J.A.S.; Oller, I.; Malato, S.; Polo-López, M.I. Synthetic fresh-cut wastewater disinfection and decontamination by ozonation at pilot scale. Water Res. 2020, 170, 115304. [Google Scholar] [CrossRef]
- Ormad, M.P.; Miguel, N.; Lanao, M.; Mosteo, R.; Ovelleiro, J.L. Effect of application of ozone and ozone combined with hydrogen peroxide and titanium dioxide in the removal of pesticides from water. Ozone Sci. Eng. 2010, 32, 25–32. [Google Scholar] [CrossRef]
- Huber, M.M.; Canonica, S.; Park, G.-Y.; Von Gunten, U. Oxidation of pharmaceuticals during ozonation and advanced oxidation processes. Environ. Sci. Technol. 2003, 37, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Benitez, F.J.; Real, F.J.; Acero, J.L.; Garcia, C. Kinetics of the transformation of phenyl-urea herbicides during ozonation of natural waters: Rate constants and model predictions. Water Res. 2007, 41, 4073–4084. [Google Scholar] [CrossRef]
- Chen, W.R.; Wu, C.; Elovitz, M.S.; Linden, K.G.; Suffet, I. (Mel) Reactions of thiocarbamate, triazine and urea herbicides, RDX and benzenes on EPA contaminant candidate list with ozone and with hydroxyl radicals. Water Res. 2008, 42, 137–144. [Google Scholar] [CrossRef] [Green Version]
- Lai, M.-S.; Jensen, J.N.; Weber, A.S. Oxidation of simazine: Ozone, ultraviolet, and combined ozone/ultraviolet oxidation. Water Environ. Res. 1995, 67, 340–346. [Google Scholar] [CrossRef]
- Nélieu, S.; Kerhoas, L.; Einhorn, J. Degradation of atrazine into ammeline by combined ozone/hydrogen peroxide treatment in water. Environ. Sci. Technol. 2000, 34, 430–437. [Google Scholar] [CrossRef]
- Arnold, S.M.; Hickey, W.J.; Harris, R.F. Degradation of atrazine by fenton’s reagent: Condition optimization and product quantification. Environ. Sci. Technol. 1995, 29, 2083–2089. [Google Scholar] [CrossRef] [PubMed]
- Legube, B.; Guyon, S.; Dore, M. Ozonation of aqueous solutions of nitrogen heterocyclic compounds: Benzotriazoles, atrazine and amitrole. Ozone Sci. Eng. 1987, 9, 233–246. [Google Scholar] [CrossRef]
- Hapeman-Somich, C.J.; Zong, G.; Lusby, W.R.; Muldoon, M.T.; Waters, R. Aqueous ozonation of atrazine. Product identification and description of the degradation pathway. J. Agric. Food Chem. 1992, 40, 2294–2298. [Google Scholar] [CrossRef]
- Acero, J.L.; Stemmler, K.; Von Gunten, U. Degradation kinetics of atrazine and its degradation products with ozone and OH radicals: A predictive tool for drinking water treatment. Environ. Sci. Technol. 2000, 34, 591–597. [Google Scholar] [CrossRef]
- Guzman-Perez, C.A.; Soltan, J.; Robertson, J.M. Kinetics of catalytic ozonation of atrazine in the presence of activated carbon. Sep. Purif. Technol. 2011, 79, 8–14. [Google Scholar] [CrossRef]
- Von Gunten, U. Oxidation processes in water treatment: Are we on track? Environ. Sci. Technol. 2018, 52, 5062–5075. [Google Scholar] [CrossRef] [PubMed]









| Chemical Name | EC50, 336 h (mg L−1) | Molecule Size (nm) | Width (Å) | Depth (Å) | Molar Mass (g moL−1) | Solubility in Water (mg L−1) | Log Kow | pKa |
|---|---|---|---|---|---|---|---|---|
| Simazine | 0.592 A | 0.784 B | 7.49 C | 10.34 C | 201.66 | 6.20(20 °C) | 2.18 | 1.62 |
| [O3] | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (mg L−1) | ||||||||||
| 5.6 | 10.5 | 15.4 | 19.7 | 24 | ||||||
| Time | [Simazine] | Reduction | [Simazine] | Reduction | [Simazine] | Reduction | [Simazine] | Reduction | [Simazine] | Reduction |
| (min) | (mg L−1) | (%) | (mg L−1) | (%) | (mg L−1) | (%) | (mg L−1) | (%) | (mg L−1) | (%) |
| 0 | 0.7 | 0 | 0.7 | 0 | 0.7 | 0 | 0.7 | 0 | 0.7 | 0 |
| 5 | 0.64 | 7.86 | 0.57 | 18.01 | 0.48 | 30.74 | 0.48 | 31.61 | 0.44 | 36.69 |
| 10 | 0.54 | 22.47 | 0.47 | 32.89 | 0.33 | 53.46 | 0.30 | 56.88 | 0.27 | 61.35 |
| 15 | 0.45 | 35.99 | 0.37 | 47.60 | 0.22 | 68.09 | 0.19 | 73.57 | 0.15 | 78.40 |
| 20 | 0.37 | 47.28 | 0.28 | 60.29 | 0.13 | 80.93 | 0.09 | 86.64 | 0.05 | 92.57 |
| pH(0 min) | 6.7 ± 0.1 | 6.7 ± 0.1 | 6.7 ± 0.1 | 6.7 ± 0.1 | 6.7 ± 0.1 | |||||
| pH(20 min) | 5.2 ± 0.1 | 5.0 ± 0.1 | 4.8 ± 0.1 | 4.5 ± 0.1 | 4.2 ± 0.1 | |||||
| AC/O3 | PAC | O3 | GAC | O3 | ||||
| Time | Reduction | Time | Reduction | Time | Reduction | Time | Reduction | |
| (min) | (%) | (min) | (%) | (min) | (%) | (min) | (%) | |
| 15 | 46 | 25 | 98 | 4320 | 50 | 25 | 98 | |
| O3/AC | O3 | PAC | O3 | GAC | ||||
| Time | Reduction | Time | Reduction | Time | Reduction | Time | Reduction | |
| (min) | (%) | (min) | (%) | (min) | (%) | (min) | (%) | |
| 10 | 51 | 39 | 96 | 10 | 52 | 10,080 | 99 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldeguer Esquerdo, A.; Varo Galvañ, P.J.; Sentana Gadea, I.; Prats Rico, D. Activated Carbon and Ozone to Reduce Simazine in Water. Water 2020, 12, 2900. https://doi.org/10.3390/w12102900
Aldeguer Esquerdo A, Varo Galvañ PJ, Sentana Gadea I, Prats Rico D. Activated Carbon and Ozone to Reduce Simazine in Water. Water. 2020; 12(10):2900. https://doi.org/10.3390/w12102900
Chicago/Turabian StyleAldeguer Esquerdo, Alejandro, Pedro José Varo Galvañ, Irene Sentana Gadea, and Daniel Prats Rico. 2020. "Activated Carbon and Ozone to Reduce Simazine in Water" Water 12, no. 10: 2900. https://doi.org/10.3390/w12102900